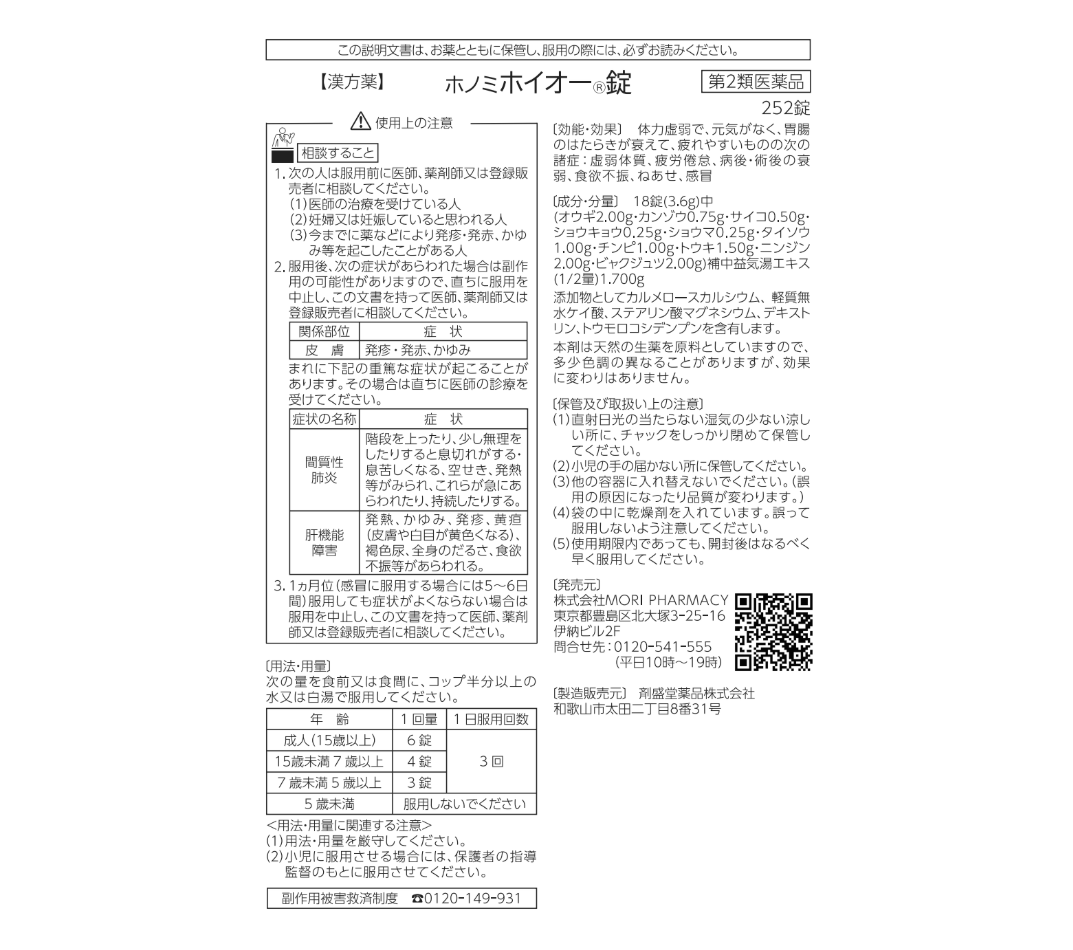
Kanpo 202 – Anti-fatigue, Exhaustion Recovery, Post-Covid Treatment
・Kanpo 202 helps improve digestive function, aiding in physical recovery and boosting energy for the user.
・Additionally, by enhancing respiratory function, the medicine is effective for those who experience shortness of breath during physical activity or are prone to catching colds.
・It can also be used for individuals with decreased physical strength, general fatigue, exhaustion, and loss of appetite.
PRODUCT DESCRIPTION
INGREDIENT
Name: KANPO 202
Dosage Form: Tablets
Pharmaceutical Classification: OTC pharmaceutical, classified as Type 2 by the Pharmaceuticals and Medical Devices Agency (PDMA) in Japan.
Product Details – Kanpo 202
Anti-Fatigue, Exhaustion Recovery, Post-Covid Treatment
Kanpo 202 helps improve digestive function, aiding in physical recovery and boosting energy for the user.
Additionally, it enhances respiratory function, making it effective for those who experience shortness of breath during physical activity or are prone to catching colds.
It is also used for individuals with decreased physical strength, general fatigue, exhaustion, and loss of appetite.
How to Use / Dosage – Kanpo 202
Anti-Fatigue, Exhaustion Recovery, Post-Covid Treatment
Usage: Take 3 times a day after meals.
| Age | Dosage per Use |
|---|---|
| Adults (15 years and older) | 6 tablets |
| 7 to 15 years old | 4 tablets |
| 5 to 7 years old | 3 tablets |
| Under 5 years old | Do not use |
<Precautions regarding dosage and usage>
- Follow the usage and dosage instructions carefully.
- For children under 15, the product should be used under the supervision of a parent or guardian.
Indications for Kanpo 202
Recommended for patients experiencing physical exhaustion, weakened digestive function, or general fatigue, accompanied by symptoms such as:
- General body weakness, fatigue, and discomfort
- Exhaustion after illness or surgery
- Loss of appetite, drowsiness, cold symptoms
Precautions When Using the Medicine
1. Cases where consultation with a doctor, pharmacist, or authorized distributor is required before using the medicine:
(1) Patients under medical treatment.
(2) Pregnant women or those who may be pregnant.
(3) Patients with a history of drug allergies.
2. Possible side effects after using the medicine:
Stop using the medicine immediately and consult a doctor, pharmacist, or authorized distributor if you experience any of the following symptoms:
| Affected Area | Symptoms |
|---|---|
| Skin | Rash, itching, swelling |
In rare cases, the following serious side effects may occur:
Stop using the medicine immediately and see a doctor if you experience any of the following symptoms:
| Condition | Symptoms |
|---|---|
| Interstitial Pneumonia | Shortness of breath when climbing stairs or engaging in physical activity; symptoms such as shortness of breath, cough, or sudden fever that appear or persist. |
| Liver Dysfunction | Fever, itching, rash, jaundice, dark urine, overall fatigue, loss of appetite, etc. |
3. If symptoms do not improve after using the medicine for approximately 1 month (1 week for cold symptoms), stop using the medicine immediately and consult a doctor, pharmacist, or authorized distributor.
In a daily dose (6 g) of this product contains 2.70 g of Hochuekkito extract of the following mixed crude drugs:
|
JP Ginseng |
2.00 g |
|
JP Atractylodes Rhizome |
2.00 g |
|
JP Astragalus Root |
2.00 g |
|
JP Japanese Angelica root |
1.50 g |
|
JP Citrus Unshiu Peel |
1.00 g |
|
JP Jujube |
1.00 g |
|
JP Bupleurum Root |
1.00 g |
|
JP Glycyrrhiza |
0.75 g |
|
JP Ginger |
0.25 g |
|
JP Cimicifuga Rhizome |
0.50 g |
(JP: The Japanese Pharmacopoeia)
Inactive ingredients: Mg metasilicate aluminate, hypromellose, crystalline cellulose, lactose, corn starch.